half life formula for first order reaction
Added Dec 9 2011 by ebola3 in Chemistry. The half-life formula for various reactions is given below.
This widget calculates the half life of a reactant in a.
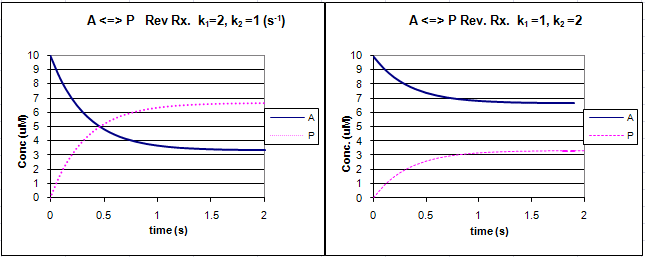
. Half-life of second order reactions shows concentration A vs. For a general reaction. The rate equation for first order reaction is k k.
This indicates that the half-life of a first-order reaction is a constant. If we set the time t equal. For first-order reactions the equation lnA.
Related
238 t 1 2 ln 2 k 0693 k. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction.
The half-life of a first-order chemical reaction A B is 10 min. Thus the graph for lnA vs t for a first-order reaction is a straight line with slope -k. TRA3C LO TRA3C5 EK Transcript.
We know that at the half-life time eqt_12 eq the concentration of the reactant will. The rate constant k for the reaction or enough information to determine it. For a first zero order reaction the half.
The half-life period of reaction is the time needed for the reactant concentration to fall to one half of its initial value. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. What is the formula for half-life of a drug.
The half-life of a chemical reaction regardless of its order is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction. Seconds what would be the second half-life assuming the reaction is either zero first or second-order. Solving for the half-life we obtain the simple relation.
If the half-life for a reaction is 20. Using the concentration-time equation for a second-order reaction we can solve for half-life. The order of the reaction or enough information to determine it.
Time t which is similar to first order plots in that their slopes decrease to zero. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant. The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure.
The half-life of a zero-order reaction the formula is given as t 12 R02k. For example after one t 12 k 0693 For a second-order reaction the half-life depends on the rate constant and the concentration of the reactant and so is expressed as half-life the. Pseudo first order reaction with examples.
The half-life formula for a reaction depends upon the order of a reaction. Half life formula for First order reaction. The half-life of a first-order reaction is given as t 12 0693k.
The half-life of a. In some cases we need to know the initial. The half-life of a second-order reaction is given by.
Half-Life of a First-Order Reaction. For a zero-order reaction the half-life equation is given as. The half-life t12 is the.
Calculate Half-life Period and its Graphical Representation 1st order reation. Half Life Calculator first order reaction input the equations calculated rate constant. Numericals on First Order Reactions.
The second half-life for the zero. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.
Half Life Of First Order Reaction Youtube
Half Life Of Zero Th 0th Order Reaction Derivation Youtube
Half Lives And Radioactive Decay Kinetics
Cbse Class 12 Chemistry Notes Chemical Kinetics
Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
First Order Reaction Definition Example Half Life Period Chemistry Notes
Rate Equation And Order Of Reaction
First Order Reaction Definition Examples And Equations
Chapter 14 Chemical Kinetics Flashcards Quizlet
Half Life Of A First Order Reaction Video Khan Academy
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
4 5 First Order Reaction Half Life Chemistry Libretexts
Answered The Half Life Of A First Order Reaction Bartleby
In The First Order Reaction 10 Of The Reactant Is Consumed In 25 Minutes Calculate I The Half Life Period Of The Reaction Ii The Time Required For Completing 87 5 Of The Reaction From Chemistry Chemical
Write The Equations Relating The Half Life Of A Second Order Quizlet

